Properties Of Fluids
Appendix B
PROPERTIES OF FLUIDS
This section contains the following:
Figure B-1. Compressibility factor Z for Pr = 0 to 0.5.
Figure B-2. Compressibility factor Z for Pr = 0 to 10.
Figure B-3. Compressibility factor Z for Pr = 0 to 40.
Table B-1. Physical constants of gases and vapors.
Table B-1 Physical Constants of Gases
| Gas | M Molecular Mass |
R Individual Gas constant |
Isentropic Coefficient |
Tc Critical Temperature |
Pc Critical Pressure (absolute) |
||||
| k for P → atm T = 273K = 492R |
°K | °R | MPa | lb/in2 | |||||
| Acetylene | C2H2 | 26.078 | 318.82 | 59.24 | 1.23 | 309.09 | 556.4 | 6.237 | 904.4 |
| Air | — | 28.96 | 287.09 | 53.35 | 1.40 | 132.4 | 238.3 | 3.776 | 547.5 |
| Ammonia | NH3 | 17.032 | 488.15 | 90.71 | 1.31 | 405.6 | 730.1 | 11.298 | 1638.2 |
| Argon | Ar | 39.994 | 208.15 | 38.68 | 1.65 | 150.8 | 271.4 | 4.864 | 705.3 |
| Benzol | C6H6 | 78.108 | 106.44 | 19.78 | — | 561.8 | 1011.2 | 4.854 | 703.9 |
| Butane-n | C4H10 | 58.124 | 143.04 | 26.58 | — | 425.2 | 765.4 | 3.506 | 508.4 |
| Butan-i | C4H10 | 58.124 | 143.04 | 26.58 | — | 408.13 | 734.6 | 3.648 | 529.0 |
| Butylene | C4H8 | 56.108 | 148.18 | 27.54 | — | 419.55 | 755.2 | 3.926 | 569.2 |
| Carbon dioxide | CO2 | 44.011 | 188.91 | 35.10 | 1.30 | 304.2 | 547.6 | 7.385 | 1070.8 |
| Carbon disulfide | CS2 | 76.142 | 109.19 | 20.29 | — | 546.3 | 983.3 | 7.375 | 1069.4 |
| Carbon monoxide | CO | 28.011 | 296.82 | 55.16 | 1.40 | 133.0 | 239.4 | 3.491 | 506.2 |
| Carbon oxysulfide | COS | 60.077 | 138.39 | 25.72 | — | 375.35 | 675.6 | 6.178 | 895.9 |
| Chlorine | Cl2 | 70.914 | 117.24 | 21.79 | 1.34 | 417.2 | 751.0 | 7.698 | 1116.3 |
| Cyanogen | C2N2 | 52.038 | 159.77 | 29.69 | — | 399.7 | 719.5 | 5.894 | 854.6 |
| Ethane | C2H6 | 30.070 | 276.49 | 51.38 | 1.20 | 305.42 | 549.8 | 4.884 | 708.2 |
| Ethylene | C2H4 | 28.054 | 296.36 | 55.07 | 1.25 | 282.4 | 508.3 | 5.070 | 735.2 |
Table B-1. Physical constants of Gases
(continued)
| Gas | M Molecular Mass |
R Individual Gas constant |
Isentropic Coefficient |
Tc Critical Temperature |
Pc Critical Pressure (absolute) |
||||
| k for P → atm T = 273K = 492R |
°K | °R | MPa | lb/in2 | |||||
| Methane | CH4 | 16.034 | 518.24 | 96.30 | 1.31 | 190.7 | 343.3 | 4.629 | 671.2 |
| Methyl chloride | CH3Cl | 50.491 | 164.66 | 30.60 | — | 416.2 | 749.2 | 6.669 | 667.0 |
| Neon | Ne | 20.183 | 411.94 | 76.55 | 1.64 | 44.4 | 79.9 | 2.654 | 384.8 |
| Nitric oxide | NO | 30.008 | 277.06 | 51.48 | 1.39 | 180.2 | 324.4 | 6.541 | 948.5 |
| Nitrogen | N2 | 28.016 | 296.76 | 55.15 | 1.40 | 126.3 | 227.3 | 3.383 | 490.6 |
| Nitrous oxide | N2O | 44.016 | 188.89 | 35.10 | 1.28 | 309.7 | 557.5 | 7.267 | 1053.7 |
| Oxygen | O2 | 32.000 | 259.82 | 48.28 | 1.40 | 154.77 | 278.6 | 5.080 | 736.6 |
| Sulfur dioxide | SO2 | 64.066 | 125.77 | 24.11 | 1.28 | 430.7 | 775.3 | 7.885 | 1143.3 |
| Propane | C3H8 | 44.097 | 188.54 | 35.04 | — | 370.0 | 666.0 | 4.256 | 617.15 |
| Propylene | C3H6 | 42.081 | 197.56 | 36.71 | — | 364.91 | 656.8 | 4.621 | 670.1 |
| Toluene | C7H8 | 92.134 | 90.24 | 16.77 | — | 593.8 | 1068.8 | 4.207 | 610.0 |
| Water vapor | H2O | 18.016 | 461.48 | 85.75 | 1.33* | 647.3 | 1165.1 | 22.129 | 3208.8 |
| Xylene | C8H10 | 106.16 | 78.32 | 14.55 | — | — | — | — | — |
*at 100 ℃
Source: VD Blatt 4, Entwurf, Jan. 1970, Berechnungsgrundlagenfürdie Durchussmessung mit Drosselgerften, Stoffwerte. By Courtesy of VDI/VDE—Gesellschaft für Mess-und Regelungstechni.
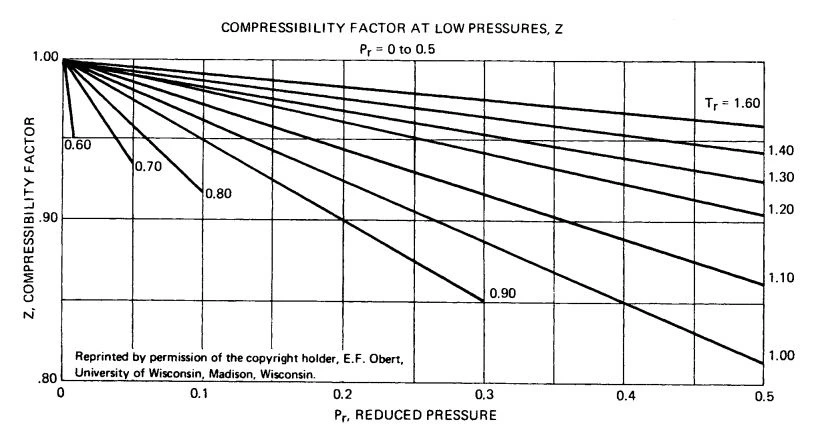
Figure B-1. Compressibility factor, Z, at low pressure for Pr=0 to 0.5
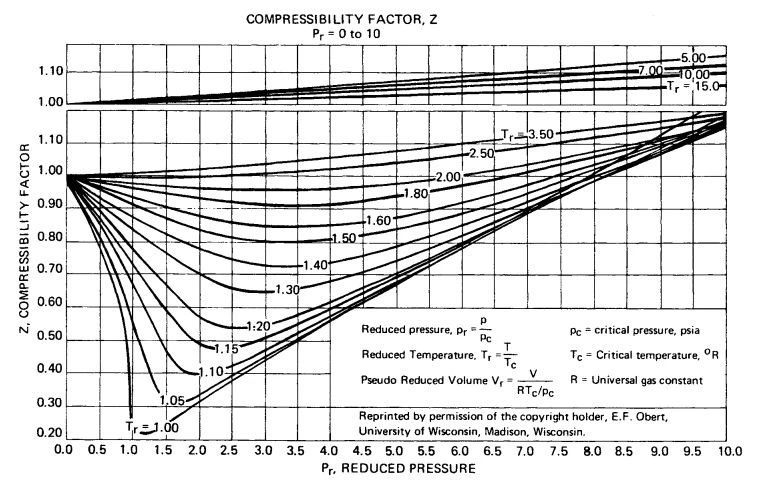
Figure B-2. Compressibility factor, Z, at low pressure for Pr=0 to 10
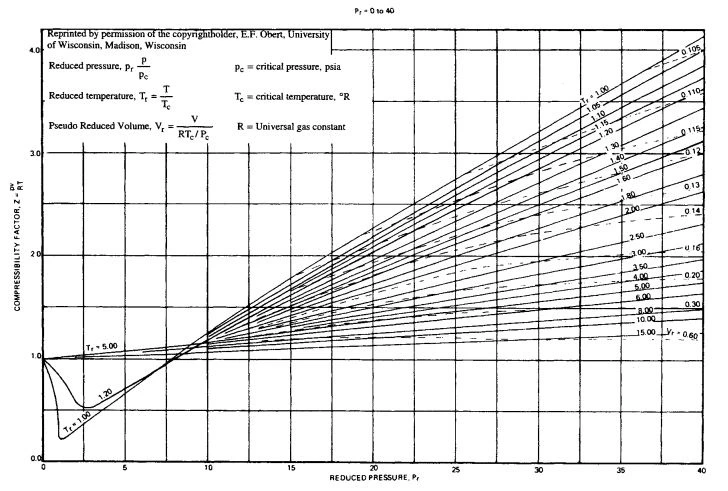
Figure B-2. Compressibility factor, Z, at low pressure for Pr=0 to 40


